https://www.timesofisrael.com/new-israeli-drug-cured-moderate-to-serious-covid-cases-within-days-hospital/
A lekovi?

- Posts : 5853
Join date : 2012-02-10
- Post n°1
 A lekovi?
A lekovi?
https://www.timesofisrael.com/new-israeli-drug-cured-moderate-to-serious-covid-cases-within-days-hospital/

- Posts : 8696
Join date : 2016-10-04
- Post n°2
 Re: A lekovi?
Re: A lekovi?
- Преко лекова се не сузбија ширење болести, као преко вакцина,
- ниједан од лекова који наводно помаже није прошао озбиљније тестове и испитивање.
- лекови који су међу првима обећавали су се касније показали као мање поуздани.

- Posts : 7775
Join date : 2017-03-14
- Post n°3
 Re: A lekovi?
Re: A lekovi?
Da, ali se drastično obara pritisak na zdravstveni sistem i smanjuje broj umrlih i onih sa težim posljedicama.

- Posts : 7775
Join date : 2017-03-14
- Post n°4
 Re: A lekovi?
Re: A lekovi?
Is Ivermectin enthusiasm research founded upon fraudulent research?🧵
— Alexandros Marinos (@alexandrosM) October 8, 2021
This investigation from the BBC, citing research by @GidMK @sTeamTraen @JackMLawrence @K_Sheldrick and @jamesheathers, claims the answer is yes.
Let's dive in and see what we find. https://t.co/jKjGuDajnq

- Posts : 8696
Join date : 2016-10-04
- Post n°5
 Re: A lekovi?
Re: A lekovi?
А лек који највише обећава, Молнупиравир, смо већ помињали. Тренутно се завршава испитивање.
Биће контроверзи око овог лека. Кошта око 30 долара да се направи, и то са малом зарадом. Продаваће се вероватно за 600 (Мартин Шкрели би ово лајковао).
Неке оптимистично процене наводе да би вакцина + лек спустиле корону на ниво грипа. Комбинација идеална за фармакомафију.

- Posts : 13817
Join date : 2016-02-01
- Post n°6
 Re: A lekovi?
Re: A lekovi?
I want to talk about some of the criticisms of our recent work on fraud in the ivermectin literature, especially the types criticisms I do see and the ones I don't see.
— Kyle Sheldrick (@K_Sheldrick) October 10, 2021
There are too many to respond to independently and I don't want to further promote bad faith takes.
1/19

- Posts : 13817
Join date : 2016-02-01
- Post n°7
 Re: A lekovi?
Re: A lekovi?
JUST IN: Merck seeks FDA emergency use authorization for molnupiravir, an experimental antiviral pill to treat Covid-19 https://t.co/P1xAvwr6XU
— CNN (@CNN) October 11, 2021

- Posts : 13817
Join date : 2016-02-01
- Post n°8
 Re: A lekovi?
Re: A lekovi?
https://www.jpost.com/health-and-wellness/coronavirus/merck-seeks-first-us-authorization-for-covid-19-tablet-681661Merck seeks first US authorization for COVID-19 tablet
An authorization from the US Food and Drug Administration could help change clinical management of COVID-19 as the pill can be taken at home.
Merck & Co Inc said on Monday it has applied for US emergency use authorization for its tablet to treat mild-to-moderate patients of COVID-19, putting it on course to become the first oral antiviral medication for the disease.
An authorization from the US Food and Drug Administration could help change clinical management of COVID-19 as the pill can be taken at home.
The treatment, molnupiravir, cut the rate of hospitalization and death by 50% in a trial of mild-to-moderately ill patients who had at least one risk factor for the disease, according to data released earlier this month.
The interim efficacy data on the drug, developed with Ridgeback Biotherapeutics, had heavily dented the shares of COVID-19 vaccine makers and set off a scramble among nations, including Malaysia, South Korea and Singapore, to sign a supply deal with Merck.
The drugmaker has a US government contract to supply 1.7 million courses at a price of $700 per course. Merck expects to produce 10 million courses of the treatment by the end of 2021.
It has also agreed to license the drug to several India-based generic drugmakers, which are expected to supply the treatment to more than 100 low- and middle-income countries.
Existing drugs from Gilead Sciences Inc's infused antiviral remdesivir and generic steroid dexamethasone are generally given only once a patient is hospitalized.
Monoclonal antibody drugs from Regeneron Pharmaceuticals Inc and Eli Lilly, which are typically infused as well, have so far seen only limited use due to the difficulty in administering them.

- Posts : 13817
Join date : 2016-02-01
- Post n°9
 Re: A lekovi?
Re: A lekovi?
Just out @LancetGH
— Eric Topol (@EricTopol) October 27, 2021
A randomized, placebo-controlled trial of fluvoxamine, a repurposed antidepressant pill (~$4/10 day course; mechanism unknown), led to improved outcomes for early treatment in high-risk patients with Covid
https://t.co/w2Z5K1YQos pic.twitter.com/R1KBDxLVdM
TOGETHER randomized trial: 11% (79/741) of the fluvoxamine group needed hospital care, vs 16% (119/756) of the placebo group (probability of superiority of 99·8%). Mortality in the per-protocol analysis: 1 for fluvox, 12 for placebo. Encouraging results! https://t.co/Lv0hw7pMFR pic.twitter.com/1vIH98nEbQ
— Paul Sax (@PaulSaxMD) October 27, 2021

- Posts : 52531
Join date : 2017-11-16
- Post n°10
 Re: A lekovi?
Re: A lekovi?

- Posts : 13817
Join date : 2016-02-01
- Post n°11
 Re: A lekovi?
Re: A lekovi?
https://www.nytimes.com/2021/10/27/health/antidepressant-fluvoxamine-covid-hospitalization.htmlA cheap antidepressant lowers the risk of Covid hospitalization, a large study finds.
A large clinical trial has found that a common and inexpensive antidepressant lowered the odds that high-risk Covid-19 patients would be hospitalized. The results, published on Wednesday, could open the door to new guidelines for the drug’s use both in the United States and globally.
The drug, fluvoxamine, has been safely prescribed for nearly 30 years as a treatment for obsessive-compulsive disorder. But when the coronavirus started spreading, researchers were drawn to the medication because of its ability to reduce inflammation, potentially allowing it to quell the body’s overwhelming response to a coronavirus infection.
Several smaller studies of fluvoxamine earlier in the pandemic showed promising results, but none was as large or persuasive as the one published on Wednesday by a group of researchers in Canada, the United States and Brazil, outside scientists said. Among nearly 1,500 Covid patients in Brazil given either fluvoxamine or a placebo, the drug reduced the need for hospitalization or prolonged medical observation by one-third, the study found. It was published in The Lancet Global Health.
Some patients struggled to tolerate the drug and stopped taking it, the study said, raising a question among outside scientists about whether they had yet identified the ideal dose. But among those who had largely followed doctors’ orders, the benefits were even more striking. In those patients, the drug reduced the need for hospitalization by two-thirds and slashed the risk of dying: One Covid patient given fluvoxamine died, compared with 12 given a placebo.
“That’s really good,” said Dr. David Boulware, an infectious disease scientist at the University of Minnesota who worked on a smaller, real-world study of the drug in Covid patients in California. Plus, he added, “it’s not a shiny new, expensive drug. The nice thing about this is it has a known safety profile.”
Beyond proper dosing, the study left other questions unresolved, scientists said. Penny Ward, a visiting professor in pharmaceutical medicine at King’s College London, noted that part of the drug’s benefit appeared to come from reducing the need for extended medical observation, which the study tracked alongside hospital admissions. And most patients in the study were unvaccinated, Professor Ward said, so it’s unclear how well the drug would work in the vaccinated.
The new study, coming nearly a year after smaller trials of the drug, was a reminder of the difficulty that many researchers have had running large tests of Covid treatments. The Biden administration has made more funding available for such trials, scientists said, but enrolling enough patients has only gotten more difficult: Most high-risk Americans are vaccinated, and vaccine-averse people may be less likely to participate in trials.
Because fluvoxamine is already approved for treating O.C.D., doctors can already prescribe it “off label” for Covid. But Dr. Boulware said that prescriptions of the drug had increased only slightly during the pandemic, unlike other repurposed drugs with far less scientific support, like hydroxychloroquine and ivermectin.
“It hasn’t really gotten any cult following,” he said.
Federal treatment guidelines say that larger trials are necessary to evaluate the use of fluvoxamine for Covid, and scientists said they expected those recommendations to change on the basis of the new study.
The new findings are also expected to boost the popularity of the drug in less wealthy countries: A 10-day course of the drug costs about $4.

- Posts : 13817
Join date : 2016-02-01
- Post n°12
 Re: A lekovi?
Re: A lekovi?
https://www.latimes.com/business/story/2021-10-27/merck-let-other-drugmakers-make-covid-pill
Pharmaceutical company Merck has agreed to allow other drugmakers worldwide to produce its COVID-19 pill in a move aimed at helping millions of people in poorer countries get access to the potentially lifesaving drug.
The Medicines Patent Pool, a United Nations-backed public health organization, said in a statement Wednesday that it had signed a voluntary licensing agreement for the drug, called molnupiravir, with Merck and its partner, Ridgeback Biotherapeutics.
The agreement will allow the Medicines Patent Pool to grant further licenses to qualified companies that are approved to make the drug. Neither drugmaker will receive royalties under the agreement for as long as the World Health Organization deems COVID-19 to be global emergency. Molnupiravir is the first pill that has been shown to treat the disease. (...)
The charity Doctors Without Borders welcomed the agreement that Merck struck to share its COVID-19 pill, but said it didn’t go far enough.
“The license excludes key upper-middle-income countries like Brazil and China from its territory, where there [is] strong, established capacity to produce and supply antiviral medicines,” said Yuanqiong Hu, a senior legal and policy advisor at Doctors Without Borders. Hu called the deal “disappointing.”

- Posts : 7775
Join date : 2017-03-14
- Post n°13
 Re: A lekovi?
Re: A lekovi?

- Posts : 13817
Join date : 2016-02-01
- Post n°14
 Re: A lekovi?
Re: A lekovi?
It seems they have given up trying to commercialize it for COVID-19:"By the time the researchers published their findings...several treatments had become available, including antiviral medications, antibody cocktails and vaccines....[T]he team has shifted focus from COVID-19...."
— ulc2020 (@ulc2020) October 27, 2021

- Posts : 13817
Join date : 2016-02-01
- Post n°15
 Re: A lekovi?
Re: A lekovi?
The Medicines and Healthcare products Regulatory Agency recommended the drug, molnupiravir, be used as soon as possible following a positive COVID-19 test and within five days of the onset of symptoms pic.twitter.com/RZWLosxCHW
— Reuters (@Reuters) November 4, 2021
The drug, to be branded Lagevrio in Britain, has been closely watched since data last month showed it could halve the chances of dying or being hospitalized for those most at risk of developing severe COVID-19 when given early in the illness https://t.co/cxcBsGv5iX
— Reuters (@Reuters) November 4, 2021
The next chapter of anti-Covid is about to start with pills that inactivate the virus, irrespective of variants. First will be Molnupiravir in a matter of weeks, then this one.
— Eric Topol (@EricTopol) November 3, 2021
Helping to get us onto an exit ramp. https://t.co/JhqWNtenA3@ScienceMagazine
Success of the Covid pill era depends on rapid tests that are widely available, free or very inexpensive, and accurate. While the pills are coming soon, the US is still woefully unprepared for their roll out.
— Eric Topol (@EricTopol) November 4, 2021

- Posts : 7775
Join date : 2017-03-14
- Post n°16
 Re: A lekovi?
Re: A lekovi?
ovaj mjesec ima još 25 dana, drago mi je što imaju preča posla

- Posts : 13817
Join date : 2016-02-01
- Post n°17
 Re: A lekovi?
Re: A lekovi?
https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidatePFIZER’S NOVEL COVID-19 ORAL ANTIVIRAL TREATMENT CANDIDATE REDUCED RISK OF HOSPITALIZATION OR DEATH BY 89% IN INTERIM ANALYSIS OF PHASE 2/3 EPIC-HR STUDY
Pfizer Inc. (NYSE: PFE) today announced its investigational novel COVID-19 oral antiviral candidate,PAXLOVID, significantly reduced hospitalization and death, based on an interim analysis of the Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients) randomized, double-blind study of non-hospitalized adult patients with COVID-19, who are at high risk of progressing to severe illness. The scheduled interim analysis showed an 89% reduction in risk of COVID-19-related hospitalization or death from any cause compared to placebo in patients treated within three days of symptom onset (primary endpoint); 0.8% of patients who received PAXLOVID
were hospitalized through Day 28 following randomization (3/389 hospitalized with no deaths), compared to 7.0% of patients who received placebo and were hospitalized or died (27/385 hospitalized with 7 subsequent deaths). The statistical significance of these results was high (p<0.0001). Similar reductions in COVID-19-related hospitalization or death were observed in patients treated within five days of symptom onset; 1.0% of patients who received PAXLOVID
were hospitalized through Day 28 following randomization (6/607 hospitalized, with no deaths), compared to 6.7% of patients who received a placebo (41/612 hospitalized with 10 subsequent deaths), with high statistical significance (p<0.0001). In the overall study population through Day 28, no deaths were reported in patients who received PAXLOVID
as compared to 10 (1.6%) deaths in patients who received placebo.

- Posts : 13817
Join date : 2016-02-01
- Post n°20
 Re: A lekovi?
Re: A lekovi?
https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/At the interim analysis, molnupiravir reduced the risk of hospitalization or death by approximately 50%; 7.3% of patients who received molnupiravir were either hospitalized or died through Day 29 following randomization (28/385), compared with 14.1% of placebo-treated patients (53/377); p=0.0012. Through Day 29, no deaths were reported in patients who received molnupiravir, as compared to 8 deaths in patients who received placebo.

- Posts : 13817
Join date : 2016-02-01
- Post n°21
 Re: A lekovi?
Re: A lekovi?
https://nova.rs/vesti/drustvo/loncar-stize-novi-lek-protiv-kovida-smanjuje-rizik-od-hospitalizacije-i-smrti/Eksperimentalni lek za lečenje koronavirusa, koji je razvila američka kompanije "Fajzer", smanjuje rizik od hospitalizacije i smrti kod pacijenata čije je zdravlje već ugroženo za 89 odsto, pokazala su klinička ispitivanja. Ministar zdravlja Zlatibor Lončar naveo je za RTS da je upravo potpisan ugovor sa predstavnicima te kompanije o distribuciji tog leka Srbiji.
Ovaj lek blokira enzim koji je potreban koronavirusu da bi se umnožavao. Kao rezultat toga-smanjuje se i smrtnost i hospitalizacija.
„Mi smo treća zemlja u svetu koja je potpisala ugovor, dogovorila se sa ‘Fajzerom’ i prvi ćemo imati taj lek koji ima 89 procenata uspešnosti za izbegavanje bolničkog lečenja i težih komplikacija“, rekao je za RTS ministar zdravlja.
Navodi da je ipak, vakcina i dalje primarno oružje u borbi sa virusom.
„Mi se ne borimo za to da dođemo u fazu da se neko razboli pa da bi se lečio već se borimo za to da ne dođe uopšte do toga nego da se ljudi vakcinišu i da ne dođu uopšte u fazu da moraju da koriste bilo koji lek. A ovo je neka naša odgovornost kao države i uspeh države koji smo obećli i koji ispunjavamo“, rekao je Lončar.

- Posts : 13817
Join date : 2016-02-01
- Post n°22
 Re: A lekovi?
Re: A lekovi?
So, not “close” at all (whatever that means; Structure? Mechanism of action?) to Ivermectin, which is a chloride channel agonist which fucks up invasive helminth nervous systems or HCQ which affects lysosomal and auto-inflammatory pathways
— Ben (@hayesy316) November 8, 2021

- Posts : 13817
Join date : 2016-02-01
- Post n°23
 Re: A lekovi?
Re: A lekovi?
A second study—the largest yet—shows that fluvoxamine is an effective treatment for COVID-19. The FDA-approved psychiatric drug interacts strongly with the sigma-1 receptor, a protein inside cells that helps regulate inflammation.https://t.co/gRmMy5tVo7
— Washington U. Med (@WUSTLmed) November 10, 2021
https://trialsitenews.com/mcmaster-together-trial-ivermectin-a-no-show-while-fluvoxamine-shows-some-promise/Recently, McMaster University's Professor Ed Mills, principal investigator of the Together trial, highlighted interim analysis results evidencing no impact of ivermectin and some other repurposed study drugs while pointing to some promise for Fluvoxamine. TOGETHER is a randomized, Adaptive Platform trial investigating several possible treatments, including ivermectin. After many therapies disappointed, Fluvoxamine, an SSRI commonly used for depression, showed some promise as a repurposed treatment.

- Posts : 13817
Join date : 2016-02-01
- Post n°24
 Re: A lekovi?
Re: A lekovi?
— Antibiotic Stewa
Excellent article
@heidiledford
'Tis the season of antiviral!#COVID19 antiviral pills "Molnupiravir & Paxlovid" :what scientists still want to know #IDTwitter #medtwitter #TwitteRx #mustread https://t.co/Wkj5s4WS6X️x
Bassam Ghanem (@ABsteward) November 10, 2021
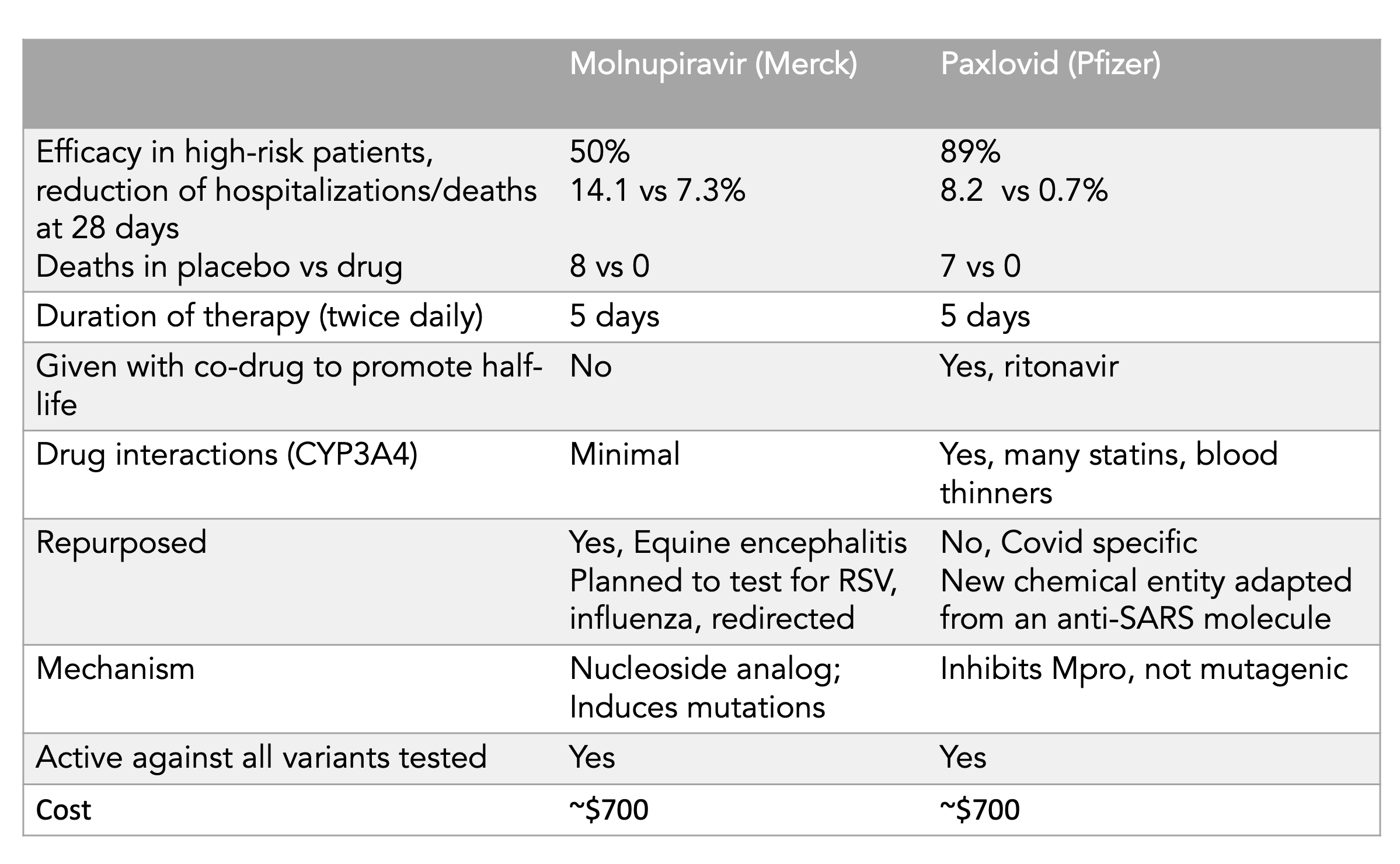
Izvor tabele je Erik Topol: https://twitter.com/EricTopol/status/1458607826515890176

- Posts : 13817
Join date : 2016-02-01
- Post n°25
 Re: A lekovi?
Re: A lekovi?
"Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries" by Stephanie Nolen and Rebecca Robbins via NYT https://t.co/YGq0XZnuxT
— Florentino Bower (@florentinobm) November 16, 2021
We have announced an important agreement with the @MedsPatentPool to expand access to our oral antiviral candidate, pending authorization or approval, in low- and middle-income countries that make up ~53% of the world’s population – more than 4B people: https://t.co/BcDTRL8HIS
— Albert Bourla (@AlbertBourla) November 16, 2021
I also want to make clear: @Pfizer will not receive royalties on sales in low-income countries and will further waive royalties on sales in all countries covered by the agreement while COVID-19 remains classified as a Public Health Emergency of International Concern by the @WHO.
— Albert Bourla (@AlbertBourla) November 16, 2021


 by kapetanm Sat Feb 13, 2021 3:42 pm
by kapetanm Sat Feb 13, 2021 3:42 pm